Quality Management
Quality management systems affect several processes, products and services worldwide.
Depending on the risk potential of the products, the requirement for “surveillance” (Audits) of the manufacturer often arises. This is then performed by a notified body (NoBo).
The ISO 9001 standard makes an important contribution to the CE conformity of products and services. For ATEX / IECEx manufacturers it can be understood as a starting point and is supplemented by the requirements of ISO 80079-34. Annex X of the Machinery Directive 2006/42 / EC also states that an effective Quality management System is justified.
In terms of product liability and associated risk minimization, an effective QMS is often indispensable.
Important items
- Contribution of the QMS to CE conformity
- Implementation of ISO 9001: 2015
- Implementation of ISO 80079-34
- Audit Program, Internal Audits, External Audits
- QMS and the safe production of products
- Appoint, empower and coach ex- authorities
- Make documented information meaningful
Topics around quality management systems
ISO 9001:2015
This International Standard is based on the principles of quality management described in ISO 9000. The descriptions include a statement on each principle, a rationale for why the principle is important to the organization, some examples of policy benefits and examples of typical measures to enhance the organization’s performance in applying the principles.
The principles of quality management are as follows:
- customer focus
- leadership
- inclusion of persons
- process-oriented approach
- improvement
- evidence-based decision-making
- relationship management
The current revision of ISO 9001 is the ISO 9001: 2015. A certificate is valid for 3 years.
ISO 80079-34 for ATEX/IECEx
Documented information acc. ISO9001:2015
The following list should give you an overview of the 21 “Documented Information” required in ISO9001: 2015.
The standard sections can be found in the brackets:
- Scope of QMS (4.3)
- Process documentation (4.4)
- QMS-Policy (5.2.2)
- Information about quality targets (6.2.1)
- The suitability of resources for monitoring and measurement (7.1.5)
- The basis for calibration or verification (7.1.5)
- Proof of competence(7.2)
- Proof that the processes are carried out as planned (8.1)
- Results of the review of customer requirements.
- Confirmation that the development requirements and any development changes have been fulfilled (8.3.2, 8.3.6)
- Results of assessments, performance and re-assessment of external providers (8.4.1)
- Characteristics of the products and services provided (8.5.1a)
- Activities to be carried out and results to be achieved (8.5.1b)
- Information needed to maintain traceability where appropriate (8.5.2)
- Review of changes in production or service provision (8.5.6)
- Authorization of the release of products and services to the customer (8.6)
- Implemented measures regarding non-compliant process results, products and services (8.7)
- Proof of the results of the monitoring and control activities (9.1)
- Internal audit programs and audit results (9.2)
- Results of Management Review(9.3)
- Non-conformities and results of corrective actions (10.2)
Ex-Authority acc. ISO 80079-34
An Ex- authority acc. ISO 80079-34 with stated and documented responsibilities shall be appointed to ensure that the following requirements are met:
a) the effective coordination of activities with regard to ex-products
(b) cooperation with the notified /certification body responsible for issuing the Ex certificate (if not issued by the manufacturer) with regard to any intended changes to the design specified in the Ex certificate and the related technical file
c) cooperation with the person responsible for the quality management system assessment
d) the authorization of releases and changes to production drawings, if appropriate
e) the authorization of special releases (see 8.7 f)
f) the accuracy of the ex-product information given to the customer in sales information and installation instructions (which must include the specific conditions of use and restrictions)
g) the effective coordination of manufacturing processes of Ex products, including the externally provided products, services and processes listed in 8.4; For manufacturers with multiple manufacturing sites, an ex-authority with the appropriate responsibilities per site must be appointed.
Risk management
Process-oriented approach
ISO 9001: 2015 requires a process-oriented approach of the organization.
In principle, it is possible to work according to the IPO model (input, processing, output). It makes sense to describe the main processes and divide them into support, core, and management processes. Recommendation: Process owners should now be determined.
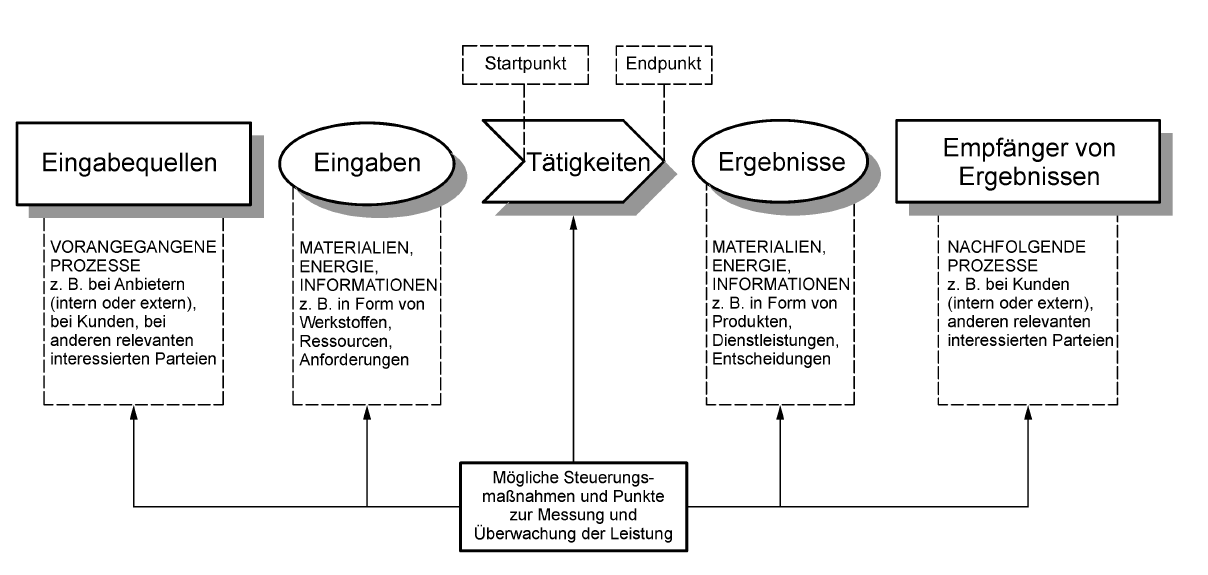
Processes of ISO 9001
Context of the organization
The organization must identify external and internal issues that are relevant to its purpose and strategic direction and that affect its ability to achieve the intended outcomes of its quality management system.
With regard to the ISO 80079-34 standard, the context of the organization must ensure that all Ex products comply with their certificate and technical documentation.
Interested parties
Due to their impact or potential impact on the organization’s ability to consistently deliver products and services that meet customer requirements and applicable regulatory and regulatory requirements, the organization shall identify the interested parties relevant to their quality management system and the requirements of those interested parties relevant to their quality management system. quality management system.
Get in contact!
I look forward to hearing from you.
